Generic Name
Composition
Therapeutic Category
Division
Amoxicillin Trihydrate
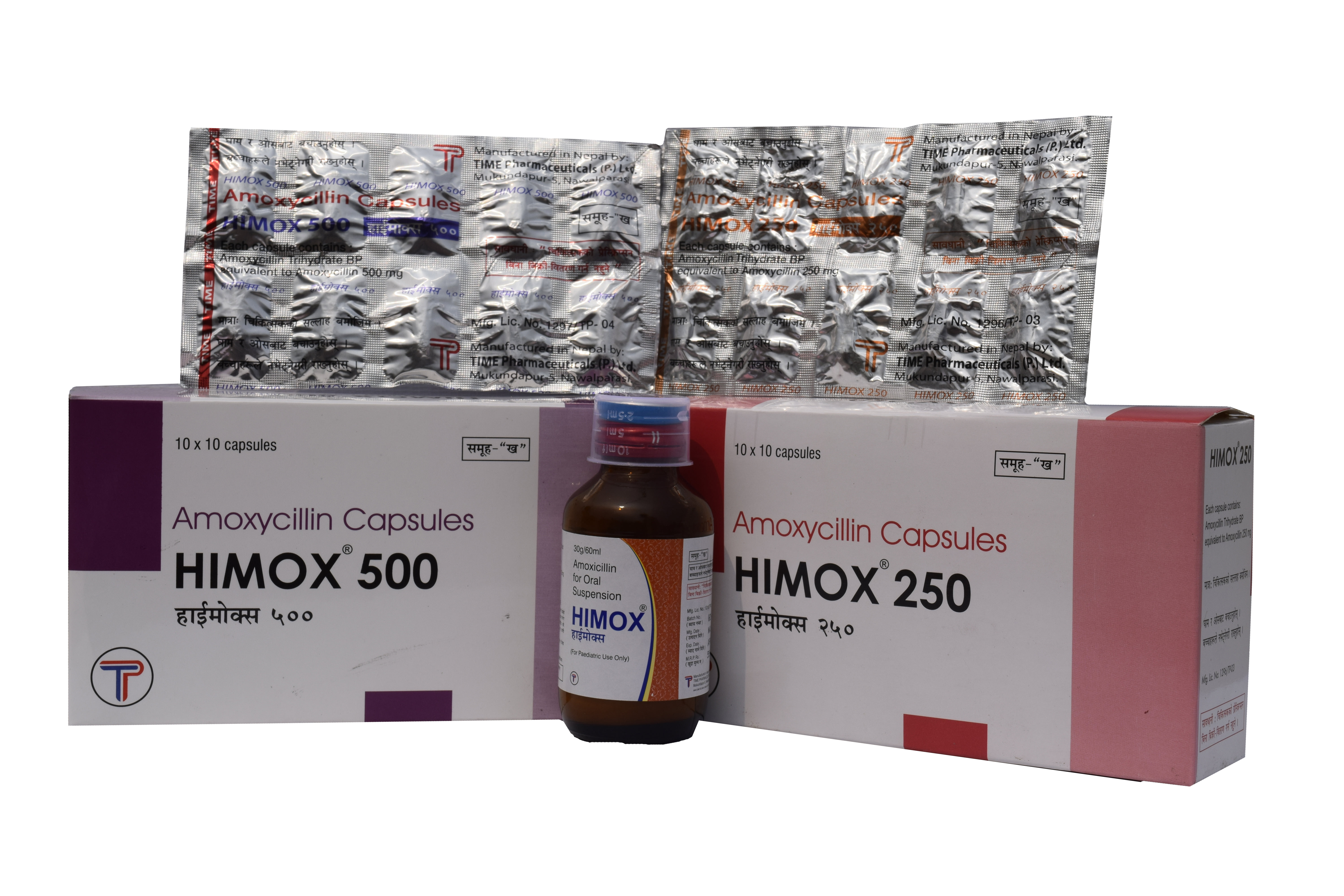
Amoxicillin Trihydrate 250mg Capsules
Amoxicillin Trihydrate 500mg Capsules
Amoxicillin Trihydrate 100mg/1ml Drops
Amoxicillin Trihydrate 125mg/5ml Dry Syrup
Penicillin Antibiotic
NEXUS
| BRAND | COMPOSITION | DESCRIPTION |
|---|---|---|
|
HIMOX 250 |
Each capsule contains: Amoxycillin Trihydrate BP equivalent to Amoxycillin 250mg |
HIMOX 250 is a size '2' hard gelatin capsule having yellow body and green cap filled with white to yellowish blend of granular powder. |
| HIMOX 500 | Each capsule contains:
Amoxycillin Trihydrate BP equivalent to Amoxycillin 500mg |
HIMOX 500 is a size '0' plain hard gelatin capsule with yellow body and green cap filled with white to yellowish blend of granular powder. |
| HIMOX Dry Syrup | Each 5 ml of the reconstituted suspension contains:
Amoxycillin Trihydrate BP equivalent to Amoxycillin 125mg |
HIMOX Dry Syrup contains white blended granules and powder filled in 78 ml overflow capacity amber colored bottle with outer ring at 60 ml volume for reconstitution. |
|
HIMOX Drops |
Each ml of Reconstituted Suspension Contains: Amoxycillin Trihydrate BP equivalent to Amoxycillin 100mg |
HIMOX Drops contain amber bottle with a label with a mark for 10 ml on the outer side filled with white blend of granular powder labeled as HIMOX PAEDIATRIC DROPS 10 ml. |
Pharmacotherapeutic Group: Penicillin antibiotics
Pharmacodynamic properties: HIMOX has bactericidal action. It inhibits the bacterial cell wall synthesis by interfering with transpeptidase enzyme. Amoxicillin is bactericidal against non β-lactamase producing G(+) organisms and selected G(-) pathogens.
Pharmacokinetic properties:
Absorption: Rapidly and completely absorbed from the GI tract. Time to peak plasma concentration: 1-2 hr
Distribution: Widely distributes into body tissues and fluids. Crosses the placenta and enters breast milk in small amounts. Plasma protein binding: approximately 20 %
Metabolism: Undergoes partial hepatic metabolism and converted to penicilloic acid.
Excretion: urine (60% as unchanged drug) and faeces. Plasma half-life: 1-1.5 hr
ENT infections, H. pylori infections, dental infections, pneumonia, respiratory tract infections, bronchitis, urinary tract infections, gonorrhea
Adult: 250-500 mg every 8 hr;
Child <10 yrs: 125-250 mg every 8 hr
<40 kg: 20-40 mg/kg daily in divided doses every 8 hours
<3 months: 30mg/kg daily in divided doses every 12 hours
PATIENT INFORMATION |
|---|
|
Administration:
Food Interaction:
Pregnancy: Category B Storage: Store capsules at or below 68°F in an air-tight container away from children. Missed dose:
|
Hypersensitivity to penicillins, cephalosporins, imipenem
Renal and hepatic disease; pregnancy; lactation; infectious mononucleosis
Serious AEs: Neuromuscular hypersensitivity; pseudomembranous colitis General AEs: Hyperactivity, agitation, insomnia, dizziness, maculopapular rash, exfoliative dermatitis, urticaria, hypersensitivity vasculitis, diarrhoea, nausea, vomiting, anaemia, thrombocytopenia, leucopenia, agranulocytosis.
Increased levels with disulfiram and probenicid. Decreased effects with tetracyclines and chloramphenicol.
Serious drug interaction: Increase effects of oral anticoagulants.
HIMOX 250: Each box is supplied with 10 strips of 10 capsules per strip
HIMOX 500: Each box is supplied with 10 strips of 10 capsules per strip
HIMOX DS: Each phial is supplied with 60 ml DS volume to be reconstituted
HIMOX Drops: Each phial is supplied with 10 ml volume to be reconstituted