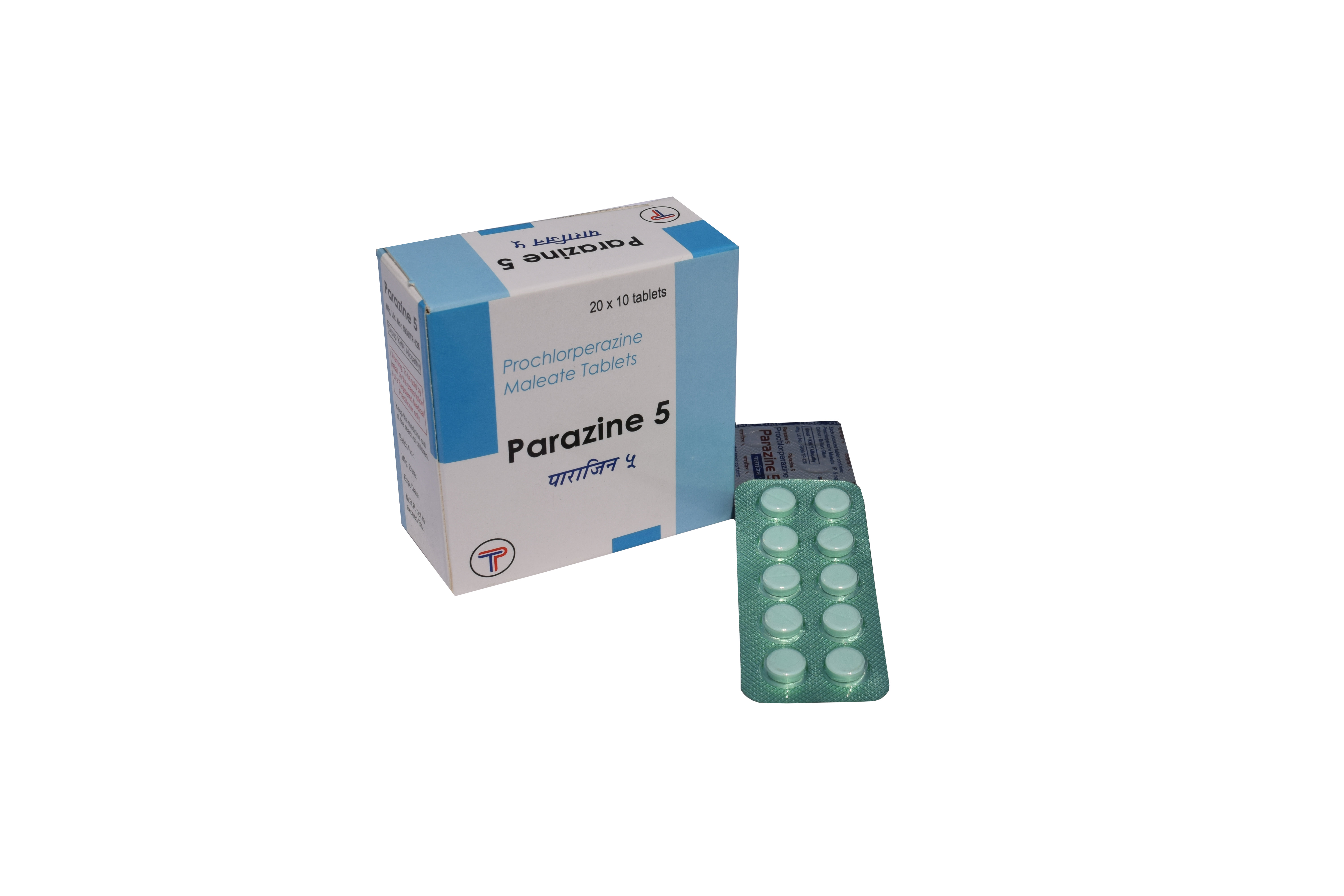
Generic Name
Composition
Therapeutic Category
Division
Prochlorparazine Maleate
Prochlorparazine Maleate 5mg Tablets
Anti-emetic
TIME
| BRAND | COMPOSITION | DESCRIPTION |
|---|---|---|
| PARAZINE |
Each uncoated tablet contains: Prochlorperazine maleate IP 5mg |
PARAZINE is a light blue, circular tablet with break-line on one side. |
Pharmacotherapeutic Group: Antiemetic and antipsychotics
Pharmacodynamic properties:
Prochlorperazine blocks both postsynaptic dopamine receptors as well as the medullary chemoreceptor trigger zone. It depresses hypothalamic and hypophyseal hormone release and posssesses α-adrenergic and anticholinergic inhibitory activity.
Pharmacokinetic properties:
Absorption: Well absorbed from the GI tract but is subject to considerable first pass metabolism from the gut wall. Bioavailability: 12.5%, onset: 30-40 min
Distribution: Extensively bound to plasma proteins, widely distributed in the body (it crosses the blood brain barrier) and its metabolites cross the placental barrier and are excreted in milk.
Metabolism: Extensively metabolised in the liver and forms N-desmethylprochlorperazine (active), excreted in the urine and bile.
Excretion: Plasma half-life is reported to be only a few hours but elimination of the metabolites may be very prolonged.
Nausea and vomiting associated with radiotherapy and chemotherapy, pre and post operation, vertigo, schizophrenia, mania
Adult: 5-10 mg twice or three times a day.
Pediatric:
Less than 2 years old: not recommended
Greater than 2 years old: 2.5-5 mg every 8-12 hr
PATIENT INFORMATION |
|---|
|
Administration:
Food Interaction: Alcohol may increase the effects of prochlorperazine. Pregnancy: Category C Nursing Mothers: Prochlorperazine maleate can pass into breast milk and may harm a nursing baby. Do not breastfeed while using prochlorperazine. Storage: Store tablets at room temperature Missed dose:
|
Children less than 2 years, lactation, hypersensitivity to phenothiazines, coma, severe CNS depression
Extrapyramidal syndrome, hypotension, epilepsy, impaired hepatic, renal, cardiovascular, cerebrovascular or respiratory function, glaucoma. May impair ability to drive or perform tasks requiring mental alertness or physical coordination. History of jaundice, parkinsonism, diabetes mellitus, hypothyroidism, myasthenia gravis, paralytic ileus, prostatic hyperplasia or urinary retention. Regular eye examinations are recommended in patients on long-term treatment
Severe AEs: Bone-marrow suppression, cardiac arrhythmias or aspiration.
Common AEs: Jaundice, cardiac arrhythmias, orthostatic hypotension, leucopenia, thrombocytopenia, dry mouth, blurring of vision, glaucoma, urinary retention, constipation, galactorrhoea, gynaecomastia, amenorrhoea
Potentiation of other CNS depressants including alcohol, sedatives, hypnotics, barbiturates, opioids, antihistamines and general anaesthetics.
Each box is supplied with 20 strips of 10 tabs packed in blisters