Generic Name
Composition
Therapeutic Category
Division
Paracetamol
Paracetamol 500mg Tablets
Paracetamol 125mg/5ml Syrup
Anti-pyretic/Analgesic
ERRA
| BRAND | COMPOSITION | DESCRIPTION |
|---|---|---|
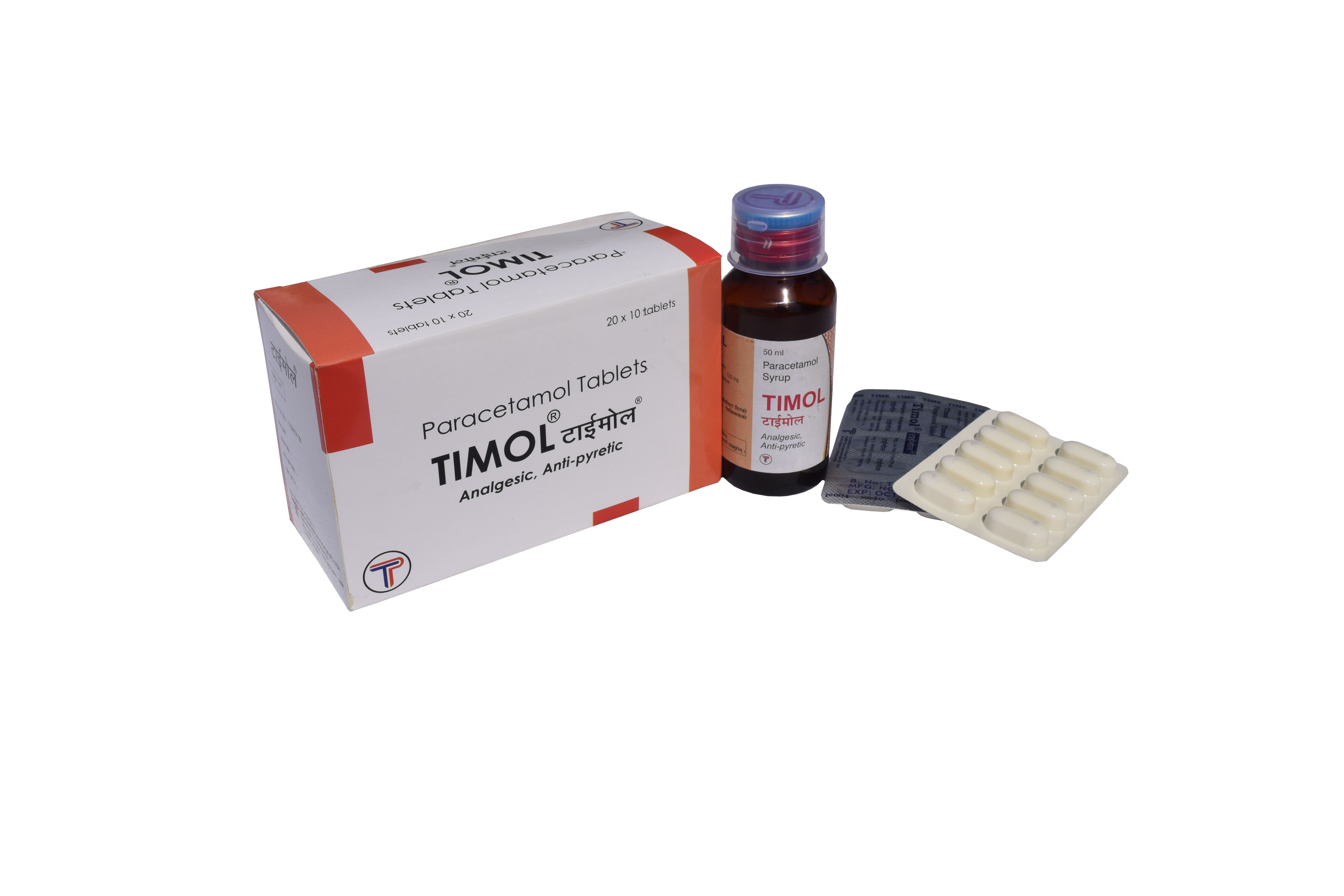
| TIMOL | Each uncoated tablet contains: Paracetamol BP 500mg |
White oblong uncoated tablet with breakline on one side |
| TIMOL Syrup | Each film coated tablet contains: Each 5 ml contains |
Light orange colored syrup 50 ml filled in amber PET bottle and sealed with PP cap. The bottle is labelled with product information. |
PATIENT INFORMATION |
|---|
|
Food Interaction: St. John’s wort may decrease effect of paracetamol. May cause serious liver side effects with alcohol. Administration: May be taken with or without food. Pregnancy:CategoryB Storage: Store at room temperature not exceeding 30°C and away from light and moisture. Missed dose: Take the missed dose as soon as you remember. Skip the missed dose if it is almost time for your next scheduled dose. Do not take extra medicine to make up the missed dose.
|
PHARMACOLOGY
Pharmacotherapeutic Group: Analgesic and Antipyretic Agent
Pharmacodynamics Properties:The exact mechanism by which acetaminophen produces its analgesic and antipyretic effects remains undefined. The primary mechanism of action is believed to be inhibition of cyclooxygenase (COX), with a predominant effect on COX-2. Inhibition of COX enzymes prevents the metabolism of arachidonic acid to prostaglandin H2, an unstable intermediate byproduct which is converted to pro-inflammatory compounds.
Pharmacokinetic Properties:
Absorption: Incomplete, depends upon dosage form. Time to peak serum: 10-60 min. May be delayed in acute overdoses. Decreased rate of absorption with food.Onset: < 1 hour; Duration: 4-6 hr
Distribution:Present in most body tissues, crosses the placenta and enters the breast milk. Protein binding: 8-43% (at toxic doses)
Metabolism:Hepatic via glucuronic and sulphuric acid conjugation.
Excretion:Plasma half-life: 2.7 hours (adults); 1.5-2 hours (infants and children); 3.5 hours (neonates). Neonates, infants and children up ≤10 years excrete less glucuronide than adults. Half-life may be longer after toxic doses. Excreted mainly via urine (2- 5% unchanged; 55% as glucuronide metabolites). Total body clearance: 18 L/hours.
INDICATIONS
Fever, mild to moderate pain
DOSAGE & ADMINISTRATION
Adults: 0.5 gm to 1 gm tablets every 4 hours when necessary (maximum 8 tablets in 24 hours).Children 6 - 12 years: 250-500 mg tablet when necessary (maximum 4 tablets in 24 hours).Children under 6 years: 1to 5 years: 120 -250 mg 3 months to 1 year: 60 to 120 mg: less than 3 months: 10mg/kg body weight
ADVERSE DRUG REACTIONS
Thrombocytopenia, leucopenia, pancytopenia, neutropenia, agranulocytosis.
Rarely, hypotension and tachycardia.
PRECAUTIONS
Patient with chronic alcoholism, known Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency, severe hypovolaemia, chronic malnutrition, renal and hepatic impairment, pregnancy and lactation.
DRUG INTERACTIONS
May reduce serum levels with anticonvulsants (e.g. phenytoin, barbiturates, carbamazepine). May enhance the anticoagulant effect of warfarin and other coumarins with prolonged use. Accelerated absorption with metoclopramide and domperidone.
CONTRAINDICATIONS
Hypersensitivity to paracetamol or any ingredient in the formulation.
PRESENTATION
TIMOL: Each box contains 20 Blisters of 10 Tablets per strip
TIMOL SYRUP: Each bottle contains 50ml of syrup